The Myth of Nuclear Weapons
Andrew McKillop
The Hiroshima Bomb
On the 6th August, 1945, US military forces dropped one special incendiary bomb on Hiroshima. According to official sources, the bomb destroyed 1.7 square miles of the city killing a disputed number of persons, due to the blast and fire deaths caused by the bomb being treated separately from the radiation deaths it caused, which were only admitted at a later date. US war officials stated that the bomb weighed “4 to 5 metric tons” and had the power of “13 to 16 kilotons of TNT”. The novelty of this bomb was that it radiated heat, light and gamma radiation whereas all classical incendiary bombs radiate only heat and light.
It was an “enhanced gamma emitting incendiary and blast weapon”. Not long after the bomb drop, US officials including White House officials said that the bomb got its energy from splitting the atom “as in the Sun”, by fission or the destructive conversion of matter into energy. It can be easily argued, using verified scientific principles that Enrico Fermi and his colleagues successfully transformed chemical energy into gamma radiation. Their bomb was a “gamma radiation weapon”, not one that “split the atom”.

Two aerial photos of atomic bomb mushroom clouds, over two Japanese cities in 1945. Atomic bomb mushroom clouds over Hiroshima (left) and Nagasaki (right)
Founding Science
Henri Becquerel, in 1886, discovered that natural uranium emits energy through a special kind of radiation, which occurs spontaneously, at a constant rate, and independently of changes in ambient factors such as pressure and temperature. Shortly afterwards, fellow French scientist Pierre Curie observed that the same radiation was emitted by pitchblende, but by unit amount of pitchblende, four times more intensely. By 1898, Curie and his wife were able to extract radium from pitchblende. The extracted and refined radium emitted vastly more energy than natural uranium.
Atomic radiation was soon classified and sorted into the 3 main categories – alpha particles, beta particles and gamma radiation. Alpha partices are roughly 4 times the size or weight of a proton. Beta particles are electrons. Gamma radiation is shorter wavelength and more dangerous to living cells organs and organisms.
Even by the 1910-1920 period intense scientific discussion and large controversy had emerged, for example the Bose-Einstein theories, on the convertibility and equivalence of mass-and-energy. In 1919, British scientist Ernest Rutherford irradiated gaseous nitrogen with moving alpha-particles, showing that Nitrogen and Oxygen can be converted or transformed, the one into the other, but for many scientists of the time, and later, this alpha particle conversion of one element to another is as questionable as later experiments. For example those by John Cockroft setting out to demonstrate that Helium can be produced from the alpha bombardment of Lithium (in 1932).
In these and other experiments, then and today, there is always the problem of “mass defects”, for example the amount of Lithium converted to Helium, in the Cockcroft experiment. The weight loss of Lithium that is bombarded should exactly equal the weight of Helium created. This did not occur. The measurement of extremely tiny changes in amu-atomic mass units is very far from being an exact science.
Energy Defects
By no later than the 1920s emerging atomic science had already spawned the idea of using radioactive heat emitted by certain minerals, and substances extracted from them, to run electric power plants. Atomic theory – the “classic Einstein theory of E = mc(2)” – says that matter and energy are interchangeable, and tiny amounts of matter contain huge amounts of energy. This is claimed to be released by fission, and not by chemical, electrical or electromagnetic conversion or transformation.
Basic scientific problems remained, and still remain. The destructibility of mass should be well demonstrated. The loss of mass should yield amounts of heat energy comparable to those predicted by Einstein’s equation, very notably.
Major issues for example include the exact weights of materials said or claimed to be transformed, and the chemical, electrical, electromagnetic or radioactive behavior of, for example, heavier atomic particles like the proton and boson (named for Bose). Scientific debate and controversy on this subject has never ceased.
Basically, if it is not possible to exactly measure the weight of these particles, their conversion by fission into radiation energy has doubt attached to it. Natural processes such as spallation in the atmosphere (nuclear transformation of for example carbon into nitrogen and oxygen) are due to cosmic-source gamma radiation. They are quantum mechanical effects, not fission effects. There is no “critical mass”. They are nothing to do with Newtonian-type “straight line” collision of particles, which is the only conceptual framework used in atomic science to supposedly cause fission and produce “mass gains and losses”.
The Fermi Experiment
US war officials took Enrico Fermi’s fission theory of shortly before 1941 seriously. Fermi had been able to release the nuclear energy of uranium through a sustained chain reaction, producing significant amounts of gamma radiation, but his “secret experiment” was not an experiment to prove that mass had been converted into heat energy. He proved that both heat energy and gamma radiation can be produced, and its energy could be transformed to other forms of energy. How he produced this gamma radiation (using large amounts of electrical energy and chemical reagents) was another subject. Both of the two US nuclear weapons dropped on Japan in 1945 were gamma radiation emitting. Their blast and incendiary effects were due to the nature of the materials used, especially uranium and the materials used in the very large bomb casings and claddings.
The Supreme War Council of Japan at first refused to surrender to the USA but soon after that the Emperor of Japan unconditionally surrendered saying that “ in view of this new type of weapon, Japan is now powerless to continue the war”.
The weight of the “Little Boy” (the bomb dropped on Hiroshima) was considerable. The “Little Boy” minus its cladding weighed at least 4 metric tons. US official accounts say it contained 63.8 kilograms of enriched uranium. Some other accounts of this bomb say it weighed about 5 tons (5000 kilograms). The “total explosive charge” inside this bomb weighed about 90 kilograms, mainly uranium and an implied 26 kilograms of plutonium, or rather plutonium and other highly radioactive materials, which were separately produced at the Hanford nuclear materials site. The bomb was also equipped with an additional “pot” of explosive materials of about 30 cms diameter and 75 cms height, called “Little Chunk”, probably of conventional incendiary materials.
It is likely or probable that “Little Boy” weighed around 5 tons, minus its bomb casing which measured about 1.5 metres by 4 metres. The overall total weight was therefore probably close to 10 tons. Today’s “strategic” nuclear weapons have similar explosive power (about 12 – 15 kilotons TNT) and are not very significantly lighter, whereas today’s “tactical” weapons, of about 4 or 5 kilotons TNT explosive power are much lighter, due for example to larger use of plutonium or similar radioactive materials.
As noted above, and using US official war data, “Little Boy” destroyed about 1.7 square miles (or 4.3 sq kms) of Hiroshima city. Taking its total weight, probably close to 10 tons, this release of destructive energy was extremely tiny by comparison with what a “true fission weapon” would have produced. Some estimates suggest that “Little Boy” may have used a total of more than 100 kilograms of enriched uranium, a highly incendiary mineral.
The “consensus” or official estimate that “Little Boy” released about 13 – 16 kilotons TNT equivalent of energy was vastly smaller than the energy that would have been released by a theoretical-only “pure fission bomb”. We can note that entirely conventional World War II ordnance such as US thermite bombs, gasoline gel bombs, and kerosene gel bombs, abundantly used for air raids on Japan and Germany typically released or had a heat of combustion of 11000 kcals per kilogram weight of the explosive materials, mainly fossil fuels.
The “Little Boy” did not yield significantly more than this, but did release gamma radiation. This is the only significant difference. Using US official data and concerning its bombing campaign on Tokyo in WWar II, US bombers dropped about 12 300 tons of non-nuclear incendiary explosives which completely burnt 53.2 sq. miles (about 136 sq kms) of the city. On this basis, about 393 tons of such conventional ordnance could have destroyed 1.7 sq miles of Hiroshima.
On a purely theoretical basis, only a few grams of Hydrogen gas (not Uranium the heaviest naturally occurring element on this planet) could have destroyed 1.7 sq miles of Hiroshima – if it had been fissioned. In other terms, a gasoline or kerosene incendiary bomb (releasing about 11 000 kcals/kg) should be exceeded in explosive power by about two billion times (2000 million times) by a “pure fission bomb”.
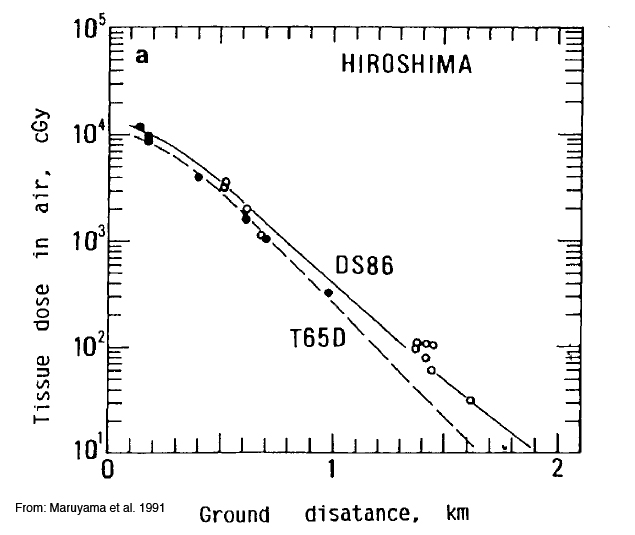
The following dosimetric diagrams show the gamma ray exposures levels at increasing distances from the epicenter of the explosions at Hiroshima and Nagasaki.
Gamma Radiation Weapons are Dangerous
This article in no way denies the danger of (especially) gamma radiation weapons, but only contradicts the claim that “conventional nuclear weapons” are fission weapons.
Gamma radiation can be produced, or rather released by a number of processes using natural materials or substances extracted from them. By comparison, if the Hiroshima atom bomb had been a “pure fission weapon” it would have needed to release the “Einstein energy” of about 0.6 grams of uranium. But the weapon, with its incendiary casing probably weighed a total of around 10 tons or 10 million grams.
Another approach is to look at the money and energy costs to produce “Little Boy”. Official US data says the total costs were about $2 billion (in dollars of 1941-45 value) but actual costs were much more than this. More important, to produce the official total of 63.8 kilograms of enriched uranium and a disputed total weight of plutonium it is likely a total of more than 9 tons of unenriched natural uranium was processed. At least 250 tons oil equivalent of energy or 375 tons of coal equivalent energy would have been needed, or more than 6 times the amount of energy released by the bomb.
The thermal, chemical and electrical energy needed to produce the so-called fission bomb was heavily disproportionate to the energy release of “Little Boy”, but the gamma radiation released was deadly to human beings, after the blast and fire effects caused by its incendiary casing or cladding.
This radiation can be released by a well-known number of other procedures – for example those used on a daily base in nuclear power plants (NPPs). For nuclear weapons that are claimed to be “instant fission weapons” however, the chemical and other energy expenditure needed to produce them is greater than the explosive energy of the weapon by a large factor.
In other words “Little Boy” got its energy from fossil fuels used to enrich uranium and produce other incendiary materials for manufacturing the bomb, which only added the novelty of gamma radiation emission. To this day of course, the nuclear weapons powers or states possessing nuclear weapons (the Security Council 5 plus 4 others) exaggerate the destructive effect and power of their so-called fission weapons. Most scientists prefer not to discuss the subject, or point to “slow controlled fission” – but no net mass gains or losses – in nuclear power plants, operated using uranium which is mined, processed, enriched, transported and disposed of using fossil fuels.
The only difference between the Hiroshima bomb and “classical” bombs was that the conventional bombs radiated light and heat, but the Hiroshima bomb also released gamma radiation. This can be obtained by other methods and procedures than “instant fission as in the Sun”, and from other materials than only uranium and plutonium, for example waste products from NPPs, medical radiotherapy wastes, industrial X-ray equipment and its wastes, and others.